Q+ cleanroom wipes and consumables are precisely measured and engineered to minimise particle release and reduce the contamination risk for critical clean spaces
From microelectronics to medicine, the cleanroom environment plays a critical role in facilitating production.
Anyone that has witnessed shelves of rejected semiconductors, or vials of desperately needed, but potentially compromised vaccines, will appreciate the need for contamination control in these spaces.
Using the right products and following the most effective cleaning procedures is key – but how can you be sure that your cleaning methods themselves are not introducing contamination and damaging the integrity of your cleanroom?
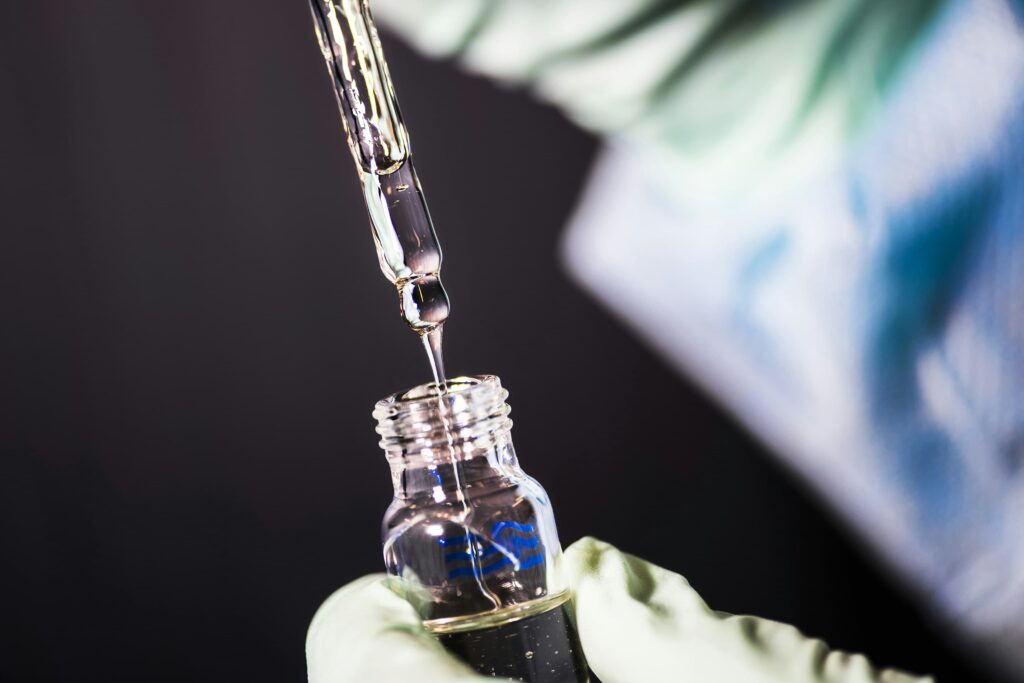
CONTROLLING CONTAMINATION
A single fibre might be the difference between a pass and fail for quality control. The implications of which could be pallets of products destroyed, with hours, or even weeks of production time wasted at huge cost. An unwanted particle in a microprocessor may result in a piece of equipment failing to operate. Contamination in a vaccine shot, could lead to increased cost, wasted product and extended production time for desperately needed medicine.
Thankfully, there are technical standards to help ensure that cleanroom operators can avoid these outcomes.
The international standard for cleanroom consumables is IEST-RP-CC004.4: Evaluating Wiping Materials Used in Cleanrooms and Other Controlled Environments – while the Institute of Environmental Sciences and Technology (IEST) is the body responsible for developing and setting the technical standards for testing the cleanliness of cleanroom consumables.
ARE YOUR CLEANROOM CONSUMABLES REALLY CLEAN?
Many suppliers to the cleanroom industry provide their products with a Certificate of Conformity (COC) to demonstrate alignment with standards outlined in their own Technical Data Sheet (TDS). This document certifies that the product is compliant to the required standards outlined by IEST and stated in the TDS. This should then be subject to the quality control and in-house testing of the supplier.
But, the COC only provides a top-level, average certification for the product. With this approach, the cleanliness of the product is stated as being in alignment with the broad potential range of cleanliness claimed in the TDS – denying the customer the clarity and opportunity to make an informed choice on the true cleanliness of the consumables. In a cleanroom environment, clarity, precision and measurement are key to cleanliness.

Q+ PRECISION-CONTROLLED PRODUCTS FOR CRITICAL ENVIRONMENTS
Gekatex Q+ cleanroom consumables (including wipes, mops and robot covers) are designed, engineered and controlled in Europe and manufactured to ensure minimum contamination. The entire process takes place in-house – this means that Gekatex’s products are both, fully customisable and fully traceable and supplied with a Certificate of Analysis.
Unlike many other manufacturers of cleanroom consumables, Q+ products are supplied with a Certificate of Analysis (COA) alongside the COC. The COA contains the test results of quality control tests, realised in accordance with IEST-RP-CC004.4 test methods. It is specific to each batch produced and it provides a transparent, comprehensive breakdown of the true contamination potential of the product. The COA empowers you to know that every product lot is – with high certainty – compliant with the technical specifications. Gekatex’s approach helps to guarantee that the consumables won’t be a determinant factor of contamination – decreasing the risk of product quality issues.
LIQUID PARTICLE COUNT

Gekatex’s dedicated lab and Liquid Particle Count (LPC) capabilities, enable them to complete their analysis in-house. Further to the LPC, the COA contains an analysis of the potential for non-volatile residues and ion contamination.
Gekatex measures the LPC to demonstrate in the most accurate way the cleanliness of the wipe. This involves first soaking the wipe in ultrapure water and agitating it to simulate use for 5 minutes. The water is then removed and analysed by the highly sensitive machine to determine the particle and fibre count. This is used to calculate the number of particles per m2 and all the information is recorded on the COA.
Get in touch to find out more about how their cleanroom consumables provide users with more clarity to help make informed decisions on the potential contamination impact of supplied products in your critical clean environment.
Find out more about our cleanroom consumables here
Contact us to speak to one of our experts!



